| D. Gering, W. Lu, K. Ruchala, G. Olivera. Anatomically Driven Deformation. International Conference on the Use of Computers in Radiation Therapy (ICCR), Amsterdam, The Netherlands, May 2010. |
| ABSTRACT: A new method of image deformation is proposed with the intent of improving the anatomical significance of the results. Instead of allowing each image voxel to move in any direction, only a few anatomical motions are permissible. The planning image and daily image are both segmented automatically. These segmentations are then analyzed to divine the values of the few parameters that govern the allowable motions. Given these model parameters, a deformation field is generated directly without iteration. This field is then passed into a pure free-form deformation process in order to account for any motion not captured by the model. Using a model to initially constrain the deformation field can help to mitigate errors. |
 |
| 1.2M |
| Poster |
| Videos: |
| Deform |
| Morph |
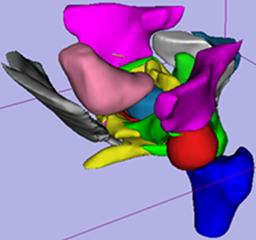
| D. Gering. W. Lu, K. Ruchala, G. Olivera. Utilizing Shape Models Composed of Geometric Primitives for Organ Segmentation. American Association of Physicists in Medicine (AAPM), Anaheim, CA, July 2009. |
| PURPOSE: Image segmentation technology can automate the task of contouring organs for radiation
treatment planning. A segmentation method using a five-layer hierarchy, proposed in AAPM 2008, propagates information
from the voxel layer up to voxel neighborhoods, then tissues, then organs, and finally organ systems. An improvement
to the organ layer is proposed that uses shape models composed of geometric primitives.
METHOD AND MATERIALS: The organ layer generally follows a sequence of six steps for each muscle and organ: (1.) Generate an ROI that limits the search space. (2.) Generate a field of candidate tissues within the ROI. (3.) On a slice-by-slice basis, recognize the object within the field that best matches expectations. (4.) Fit a geometric shape model to the cross-section of the object on each slice. (5.) Smooth the shape parameters over all slices. (6.) Refine the object boundary by reconciling shape and image data. Consider how to define the shape model used by the fourth step. Use an ellipse for the bladder, rectum, and penile bulb. For the prostate, use a large circle with its bottom quarter cut off, and two small side-by-side circles with radii half that of the large circle lowered into position. For seminal vesicles, use a horizontal ellipse for the caudal-most centimeter, which then splits into two ellipses that are permitted to tilt and slide laterally and posteriorly. RESULTS: The segmentation method using geometric shape models has been trained and tested on 50 prostatic datasets. Processing time for volumes with roughly 90 slices, and 256x256 pixels per slice, is 40 seconds on a standard PC, without any human interaction. The segmentation results were verified by trained experts and redeemed to be acceptable. CONCLUSION: Shape models composed from geometric primitives are a useful method of regularizing the segmentation of muscles and organs. |
 |
| 6K |
| Poster |
| Talk |
| Videos: |
| #1-2D |
| #1-3D |
| #2-2D |
| #2-3D |
| #3-2D |
| #3-3D |
| #4-2D |
| #4-3D |

| D. Gering. W. Lu. An Automatic Contouring Method that Combines Rule-based, Atlas-based, and Mesh-based Approaches. American Association of Physicists in Medicine (AAPM), Houston, TX, July 2008. |
| PURPOSE: Image segmentation technology can automate the task of contouring organs at risk for radiation
treatment planning. While automatic methods tend to be rule-based, atlas-based, or mesh-based, a combination of all three
approaches is proposed.
METHOD AND MATERIALS: Model-based segmentation can be initialized by automatically positioning the adaptable meshes relative to the scan. The entire scene is segmented in order to recognize the approximate organ locations. Segmentation is accomplished by a 5-layer hierarchy that propagates information from the voxel layer up to voxel neighborhoods, then tissues, then organs, and finally organ systems. The voxel layer treats each voxel as an independent statistical event. Bayesian classification uses an atlas in the form of a spatially varying prior constructed from training data. The classification is adaptive within an Expectation-Maximization framework that iteratively performs segmentation and model-estimation simultaneously.The neighborhood layer introduces local contextual constraints using Markov random fields. The tissue layer corrects mistakes using a series of logical rules. The organ layer divides tissue into different organs, or stitches different tissues together to form an organ. Organ cross-sections are segmented in order by quantitative confidence measures. The systems layer manipulates geometric primitives that have been fit to organs in order to reconcile overlaps. RESULTS: The automatic initialization of meshes has been trained and tested on 5 prostatic datasets. Processing time for volumes with roughly 60 slices, and 256x256 pixels per slice, is 40 seconds on a standard PC, without any human interaction. The segmentation results from the same 5-layer hierarchy were verified by the trained experts and redeemed to be acceptable. CONCLUSION: A 5-layer hierarchy can perform both recognition and delineation, seeing the big picture as well as the details. Such a framework where local decisions are supported by global properties can be useful in addressing inconsistent rectum contents. |
 |
| 7K |
| Poster |
Links